A patient group representing several British victims has launched legal action against the Spanish government, accused of failing to protect people from the potentially fatal side effects of one of the country’s most popular painkillers, which has been implicated in a series of serious illness and death.
Metamizole, a drug commonly sold in Spain under the brand name Nolotil, is banned in several countries, including Britain, the United States, India and Australia. This can cause a condition called agranulocytosis, which reduces white blood cells, increasing the risk of a life-threatening infection.
THE Association of Patients Affected by Drugs (ADAF) claims that adverse drug reactions have led to sepsis, organ failure and amputations. It identified around 350 suspected cases of agranulocytosis between 1996 and 2023, including those of 170 Britons living in Spain or on holiday there.
ADAF is reviewing more than 40 deaths in which it considers the drug may have caused or contributed to the death. The patient group says case reports, including a 2009 study, suggest the British population may be more sensitive to the drug’s side effects, but this has not been confirmed by an independent scientific study.
The group is demanding a drug investigation and new controls. She filed her appeal on November 14 before the National Court in Madrid. Cristina García del Campo, founder of the organization, said: “This drug has destroyed people’s lives and it should now be withdrawn. One lady took three tablets and had part of her feet and several fingers amputated. Even if it doesn’t kill you, once you have sepsis, your body is no longer the same.
Metamizole was first produced commercially in Germany in 1922 and was available worldwide until it was discovered that there was a risk of causing agranulocytosis. It has been withdrawn in around 30 countries, but is still widely available across the EU.
Studies have shown a dramatic variation in the estimated incidence of agranulocytosis in response to the drug, from approximately one in 2,000 to less than 1.1 per million users. A Report from the European Medicines Agency in December 2018, it was suggested that “the potential to induce agranulocytosis may be associated with genetic characteristics of certain populations”.
García del Campo, a translator from Jávea to Alicante, began investigating when one of her clients, an Irishman, fell seriously ill, with infections that ravaged his body. He was admitted to the Dénia City Hospital and died on November 18, 2017 from sepsis and multiorgan failure.
She said: “I was the last person with him and I held his hand. The whole time I was with him, I kept asking, “Why is this happening?” How does someone go from being unwell to having this terrible infection? »
She began compiling recent worrying reports she had heard locally regarding agranulocytosis and sepsis. One night in December 2017, she spread out all the files and medical notes she had collected on six cases. Then it hit her: everyone involved was taking metamizole.
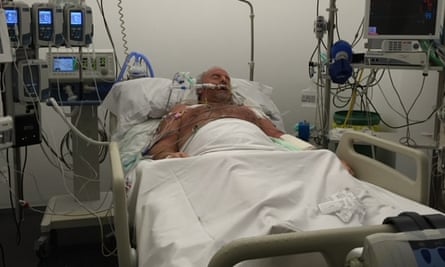
One of the patients in García del Campo’s file, Paddy Clancy, 80, a British expatriate living in Jávea, said last week that he nearly died after being given metamizole following surgery. shoulder in September 2017. He became so ill that doctors put him into an induced coma to give his body the best chance of fighting off the infection.
Clancy said: “My wife was told, ‘Her kidneys are contracting and her organs are shutting down.’ They thought I might not make it through the weekend. The family learned that his body’s white blood cells, which usually fight infections, had been severely depleted.
Clancy came out of the coma after 39 days. He had lost 22 kg and could no longer stand, but gradually recovered. His records confirm the illness that nearly killed him: “metamizole-associated agranulocytosis.”

García del Campo discovered many similar cases. An Irish holidaymaker, William Smyth, 66, died in April 2016 from multiple organ failure after being prescribed Nolotil for shoulder pain. Mary Ward, 59, who lived in Spain, died in March 2006 after being given Nolotil following an operation in Marbella and contracting agranulocytosis and complications. In another case, a woman in her 60s required an amputation after taking the drug and developing sepsis.
Agranulocytosis is said to be an extremely rare reaction to metamizole, but García del Campo was soon inundated with reports. She said the cases appeared to show the British and Irish community were more susceptible. A study in April 2009 had reviewed 13 cases of agranulocytosis involving dipyrone (another name for metamizole) at the Costa del Sol Hospital in Marbella, including five cases involving Britons. It concluded: “Dipyrone-related agranulocytosis is a more common adverse reaction in (the) UK population, and its use should be avoided. »
ADAF presented in its case evidence from a regional health official who claimed that a local study in five health departments in Spain had “surprisingly” found that the British population had sensitivity to metamizole from order of “80 to 120 times higher” than that of metamizole. the Spanish. This report has not been published and there is no comprehensive, robust epidemiological evidence to date to support the theory that the British or Irish may be more susceptible to the side effects of the drug.
In April 2018, Lorna Vincent, 75, who lived in Spain, was hospitalized in Benidorm to undergo an operation to repair a small hole in her intestine. His daughter, Kim Glasby, 59, from Brixham, Devon, said the operation appeared to be a success and she was given metamizole as a painkiller, but then became seriously ill. Glasby said: “The surgeon told me she didn’t have enough white blood cells and was not responding to painkillers. They didn’t know what to do.

Vincent died on April 18; her family learned that she was suffering from multiple organ failure. Glasby believes, in light of other cases, that a reaction to metamizole was to blame and is now trying to obtain all of his mother’s medical records.
In October 2018, less than a year after García del Campo’s campaign began, the Spanish Medicines and Health Products Agency, AEMPS, published new guidelines for metamizole. It recommends avoiding its use among tourists (described as the “floating population”) and informing patients of the symptoms of agranulocytosis.
García del Campo says the new guidelines have been widely violated. She said patients were not warned of the risks and the drug could be obtained without a prescription.
Carla Cardwell, 41, originally from the UK and now living in Gibraltar, gave birth to her son, Caiden, in December 2019 by cesarean section just over the Spanish border in the town of La Línea. He was prescribed metamizole.
She became so ill in January 2020 that she went to a nearby A&E unit in Gibraltar. “They thought I had cancer because I didn’t have any white blood cells,” she said. “The consultants said, ‘We don’t know what’s wrong. You have the blood results of someone with cancer, but you do not have cancer.
A senior doctor who reviewed her case asked her if she had recently taken metamizole. She said yes and that she had been diagnosed with agranulocytosis. She was told she needed granulocyte colony-stimulating factor (G-CSF) injections to regenerate her bone marrow.
She said: “My pancreas, liver and intestines were all infected. The injections had to be flown to the hospital and were the worst part. I could even feel the pain in my eye sockets. I will be forever grateful to the consultant who saved my life. She underwent therapy for post-traumatic stress after her ordeal. Her medical records indicate that she suffered from agranulocytosis, with metamizole being the suspected cause.
ADAF’s legal action, against the Spanish Ministry of Health and AEMPS, claims that the drug is being offered to patients without proper controls. He calls for a ban on giving this drug to citizens of countries where metamizole has been withdrawn and a new analysis of the risk factors linked to agranulocytosis. It also states that the drug information sheet needs to be revised.
Francisco Almodóvar, the lawyer representing ADAF, said: “We have testimonies from British people telling their stories. We can support the evidence with clinical records. This is a very important public health problem.
The company that makes Nolotil, Boehringer Ingelheim, said: “Nolotil is a prescription medication. Its ingredient, metamizole, has been used by patients for almost 100 years, with an established and well-known safety profile.
“Agranulocytosis is described as a very rare adverse reaction in current prescribing information. This is an adverse reaction that has been known for decades and available scientific information has confirmed the well-known safety profile of metamizole.
“The side effect of agranulocytosis is addressed in the current product information. The current prescribing information adequately addresses current knowledge about the risks associated with the use of Nolotil. We welcome any information that helps us improve the benefit-risk profile and safety of use of our medicines.
AEMPS returned the Observer to the Spanish Ministry of Health, which did not respond to a request for comment.



